
(A branch’s terminal doesn’t need an extra hydrogen added in the calculation because an hydrogen at where the branch attached to must have been replaced, which is true for a branch terminated with any element.) Each of the two terminal carbons needs one extra hydrogen – that is why 1 is added to the formula.(This is also true if a carbon is added into the structure, whether it is inserted to a backbone chain, attached to a terminal to replace an H, or branched out from a carbon to replace an H.)
 Except the terminal carbons, each of the carbon chained to the structure with a single bond requires a pair of hydrogen atoms attached to it - that is why the number C is in the formula, which actually represents the number of hydrogen pairs requires for that number of carbons in a saturated structure. Each of the terms on the RHS can be explained, respectively, as follows: Where C, N, H and X represent the number of carbon, nitrogen, hydrogen and halogen atoms, respectively. For non-hydrocarbons, the elements in a pair can include any elements in the lithium family and the fluorine family in the periodic table, not necessary all H's.Ī popular form of the formula is as follows: In either case, oxygen and other divalent atoms do not contribute to the degree of unsaturation, as 2 − 2 = 0.įor hydrocarbons, the DBE (or IHD) tells us the number of rings and/or extra bonds in a non-saturated structure, which equals to the number of hydrogen pairs that are required to make the structure saturated, simply because joining two elements to form a ring or adding one extra bond in a structure reduces the need for two H's.
Except the terminal carbons, each of the carbon chained to the structure with a single bond requires a pair of hydrogen atoms attached to it - that is why the number C is in the formula, which actually represents the number of hydrogen pairs requires for that number of carbons in a saturated structure. Each of the terms on the RHS can be explained, respectively, as follows: Where C, N, H and X represent the number of carbon, nitrogen, hydrogen and halogen atoms, respectively. For non-hydrocarbons, the elements in a pair can include any elements in the lithium family and the fluorine family in the periodic table, not necessary all H's.Ī popular form of the formula is as follows: In either case, oxygen and other divalent atoms do not contribute to the degree of unsaturation, as 2 − 2 = 0.įor hydrocarbons, the DBE (or IHD) tells us the number of rings and/or extra bonds in a non-saturated structure, which equals to the number of hydrogen pairs that are required to make the structure saturated, simply because joining two elements to form a ring or adding one extra bond in a structure reduces the need for two H's. 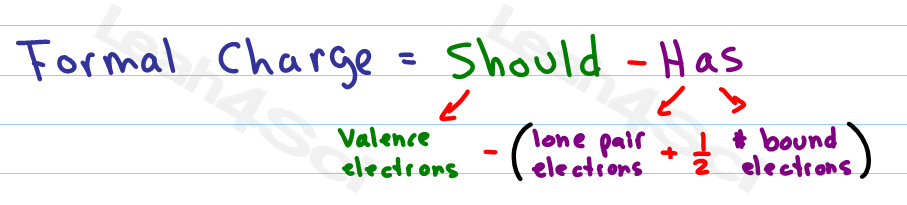
Where C = number of carbons, H = number of hydrogens, X = number of halogens and N = number of nitrogens, gives an equivalent result. The formula for degree of unsaturation is:ĭ U = 1 + 1 2 ∑ n i ( v i − 2 )



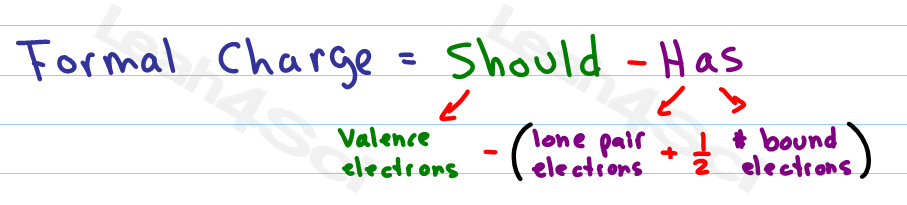


 0 kommentar(er)
0 kommentar(er)
